Fentanyl
Dr. Joseph E. Graas, Scientific Director
Dr. Edward Moore, Medical Director
The history of the opiates date back more than 2000 years with the use of the naturally occurring alkaloid analgesic from the opium poppy seed Papaver Somniferum. The class of organic compounds is called alkaloids and contains nitrogen, carbon, and oxygen arranged in ring structures. They are mostly produced by plants, animals and microbiological life; however, can also be synthesized. The term analgesic refers to the compound’s ability to block pain. However, all alkaloids are not analgesics. Examples of other alkaloids include neuroreceptors such as serotonin, ephedrine (the decongestant), nicotine, and cocaine.
The opiates that are going to be considered for comparison are purposed according to their analgesic strengths and listed in Table 1. This table contains the natural opiates (morphine and codeine) the semi-synthetic opiates (hydrocodone, hydromorphone, oxycodone, and oxymorphone) and finally the synthetic drug fentanyl. Table 1 also lists the analgesic pain reduction of the different compounds. As it turns out, the stronger the analgesic potency, the greater the abuse potential of the drug.
Fentanyl is an extremely fast acting and potent synthetic opiate that was originally discovered and introduced in 1963 as an analgesic supplement. The structure of fentanyl is distinctly different when compared to the chemical structure of Morphine or the other opioids. The potency of fentanyl is 100 to 200 times greater than Morphine and does not even have a close second when compared to the other Morphine analogues. The absorption of fentanyl is fast because of its high solubility in fatty tissue, and for the same reason, quickly crosses the blood brain barrier. The power as a high potency analgesic and the quick absorption makes fentanyl a good candidate for pain relief, and with the application of a time release delivery, fentanyl is very effective for relief of pain for extended periods of time. Please refer to the two following structures for the difference in the two compounds.
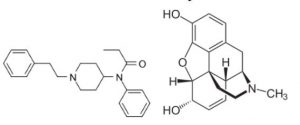
Chemical Structure of Fentanyl Compared to the Chemical Structure of Morphine
Please see table 1 for the comparison of the analgesic nature of the opioids when compared to morphine. All of the opiate molecules are agonists and all of them have the principle mu receptor as the active binding site. They are also all effectively blocked at these receptor sites by the antagonists naloxone, naltrexone and nalorphine. These compounds block the binding site, which prevents the opiate from physically getting close enough to activate the area. This binding is concentration dependent and both agonist and antagonist compete for the site
Table 1
Analgesic Potency of the Common Opiates
| Drug | Analgesic Potency (Morphine = 1) |
| Fentanyl | 100-200 |
| Hydrocodone | 1-2 |
| Hydromorphone | 7-10 |
| Morphine | 1 |
| Codeine | 0.1 |
| Oxycodone | 1-2 |
| Oxymorphone | 8-15 |
Fentanyl, due to it extremely different structure, is not detected by routine opiate urine screens. Urine screening methodology is almost universally done by an immunological method where an antibody is raised against the parent drug that is trying to be detected. In the case of opiates, that is morphine. Thus, as opioid drugs diverge (or become different in structure from morphine), the less likely they are to show positive on a routine drug screen. Other opioids not detected by the opiate screen include oxycodone (OxyContin), methadone, and buprenorphine (Suboxone).
This fact is of major clinical significant when these drug screens are used in clinical practice. Especially when considering that OxyContin is a major drug of abuse and both methadone and Suboxone are frequently diverted to the street. This limitation can be eliminated by using specific urine screens developed to detect these outlier drugs by raising antibodies against these drugs.
Clinical uses of drug screens are only as good as the ability to interpret them and this requires knowledge of their limitations. The potential for false negatives is one such limitation. When drugs, medications, or other substances are structurally similar, these can bind to the test reagent’s antibodies and cause a false positive result. It is important for clinic staff to understand how the medications may or may not react with testing. Both false negatives and false positives can be further defined by a specific class test to identify the target molecule, typically by either a GC/MS or HPLC method.